Multiple Choice
Given the data in the table below,ΔH°rxn for the reaction SO3 (g) + H2O (l) → H2SO4 (l)
Is ________ kJ. 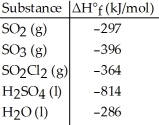
A) -132
B) 1496
C) 704
D) -704
E) -2.16 × 103
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q88: The ΔE of a system that releases
Q89: The temperature of a 35.1 g sample
Q90: A 3.00 L pitcher of sweetened ice
Q91: At what velocity (m/s)must a 417.3 g
Q92: Calculate the work (kJ)done during a reaction
Q94: Given the data in the table below,ΔH°<sub>rxn</sub>
Q95: For the following reactions,the ΔH°<sub>rxn</sub> is NOT
Q96: The internal energy of a system _.<br>A)is
Q97: For the following reactions,the ΔH°<sub>rxn</sub> is NOT
Q98: The kinetic energy of a 10.3 g