Multiple Choice
The temperature of a 35.1 g sample of iron increases from 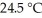 to
to 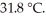 If the specific heat of iron is
If the specific heat of iron is 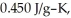 how many joules of heat are absorbed?
how many joules of heat are absorbed?
A) 115 J
B) 0.0936 J
C) -115 J
D) 0.722 J
E) 3.29 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q84: Calculate the kinetic energy in joules of
Q85: The value of ΔH° for the following
Q86: All of the following statements are true
Q87: For the combustion reaction of methane,ΔH°<sub>f</sub> is
Q88: The ΔE of a system that releases
Q90: A 3.00 L pitcher of sweetened ice
Q91: At what velocity (m/s)must a 417.3 g
Q92: Calculate the work (kJ)done during a reaction
Q93: Given the data in the table below,ΔH°<sub>rxn</sub>
Q94: Given the data in the table below,ΔH°<sub>rxn</sub>