Multiple Choice
Given the data in the table below,ΔH°rxn for the reaction 3Cl2 (g) + PH3 (g) → PCl3 (g) + 3HCl (g)
Is ________ kJ. 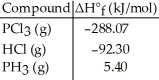
A) -385.77
B) -570.37
C) 570.37
D) 385.77
E) The ΔH°f of Cl2 (g) is needed for the calculation.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: When work is done on a system,w
Q2: The value of ΔH° for the reaction
Q4: Calculate the kinetic energy in joules of
Q5: The value of ΔE for a system
Q6: Calculate the value of ΔE in joules
Q7: Work equals mass times distance.
Q8: For the following reactions,the ΔH°<sub>rxn</sub> is NOT
Q9: Energy units include watts,volts,and newtons.
Q10: The value of ΔH° for the reaction
Q11: The value of ΔH° for the reaction