Multiple Choice
Which electron configuration represents a violation of the Pauli exclusion principle?
A) 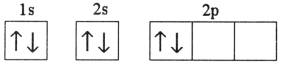
B) 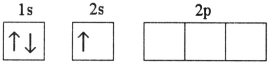
C) 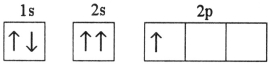
D) 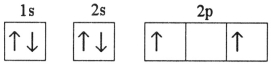
E) 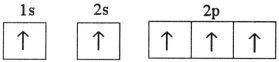
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q155: An electron in a Bohr hydrogen atom
Q156: The ground state electron configuration of copper
Q157: Which one of the following orbitals can
Q158: If a hydrogen atom electron jumps from
Q159: The angular momentum quantum number is 3
Q161: The wavelength of light that has a
Q162: The de Broglie wavelength of a 0.02900
Q163: Calculate the energy (J)found in one photon
Q164: A 4p<sub>z </sub>orbital in a many-electron atom
Q165: The ground-state electron configuration of V is