Multiple Choice
Calculate the energy (J) found in one photon of visible light if the wavelength is 589 nm.
A) 3.37 × 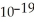
B) 1.17 × 10-31
C) 1.17 × 10-22
D) 3.37 × 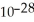
E) 2.96 × 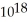
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q158: If a hydrogen atom electron jumps from
Q159: The angular momentum quantum number is 3
Q160: Which electron configuration represents a violation of
Q161: The wavelength of light that has a
Q162: The de Broglie wavelength of a 0.02900
Q164: A 4p<sub>z </sub>orbital in a many-electron atom
Q165: The ground-state electron configuration of V is
Q166: Which one of the following is correct?<br>A)ν
Q167: Ham radio operators often broadcast on the
Q168: What is the wavelength of light (nm)that