Multiple Choice
Which electron configuration represents a violation of the Pauli exclusion principle?
A) 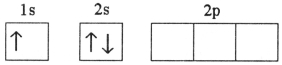
B) 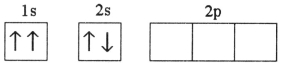
C) 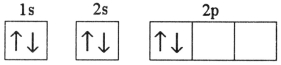
D) 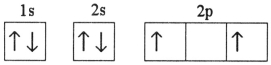
E) 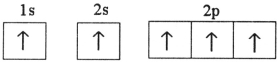
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q58: The symbol for the spin magnetic quantum
Q59: The ground state electron configuration of scandium
Q60: The square of Schrodinger's wave equation is
Q61: There are _ orbitals in the second
Q62: The energy (J)required for an electronic transition
Q64: Which is the correct electron configuration for
Q65: The frequency of a photon that has
Q66: An FM radio station broadcasts electromagnetic radiation
Q67: Which set of three quantum numbers (n,l,m<sub>l</sub>)corresponds
Q68: Electromagnetic radiation with a wavelength of 640