Multiple Choice
Electromagnetic radiation with a wavelength of 640 nm appears as orange light to the human eye.The frequency of this light is ________ s-1.
A) 4.688 × 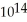
B) 4.688 × 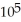
C) 1.920 × 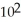
D) 1.920 × 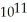
E) 2.133 × 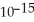
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q63: Which electron configuration represents a violation of
Q64: Which is the correct electron configuration for
Q65: The frequency of a photon that has
Q66: An FM radio station broadcasts electromagnetic radiation
Q67: Which set of three quantum numbers (n,l,m<sub>l</sub>)corresponds
Q69: In a hydrogen atom,an electron in a
Q70: The 3p subshell in the ground state
Q71: The elements in the _ period of
Q72: There are _ orbitals in the third
Q73: High energy and low wavelength light has