Multiple Choice
The Lewis structure of the CO32- ion is ________.
A) 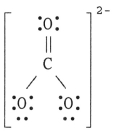
B) 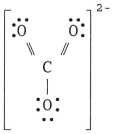
C) 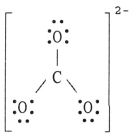
D) 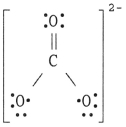
E) 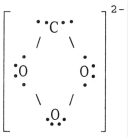
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: What species has the electron configuration [Ar]3d<sup>2</sup>?<br>A)Mn<sup>2+</sup><br>B)Cr<sup>2+</sup><br>C)V<sup>3+</sup><br>D)Fe<sup>3+</sup><br>E)K<sup>+</sup>
Q32: The Lewis structure of PF<sub>3</sub> shows that
Q33: In the resonance form of ozone shown
Q34: In the Lewis symbol for a nitrogen
Q35: The _ ion is represented by the
Q37: What is the electron configuration for the
Q38: The strength of a _ bond is
Q39: Which of the following noble gas electron
Q40: A positive change in bond enthalpy is
Q41: As the number of covalent bonds between