Multiple Choice
In the resonance form of ozone shown below,the formal charge on the central oxygen atom is ________. 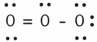
A) 0
B) +1
C) -1
D) +2
E) -2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q28: How many different types of resonance structures
Q29: Which two bonds are most similar in
Q30: Of the atoms below,_ is the most
Q31: What species has the electron configuration [Ar]3d<sup>2</sup>?<br>A)Mn<sup>2+</sup><br>B)Cr<sup>2+</sup><br>C)V<sup>3+</sup><br>D)Fe<sup>3+</sup><br>E)K<sup>+</sup>
Q32: The Lewis structure of PF<sub>3</sub> shows that
Q34: In the Lewis symbol for a nitrogen
Q35: The _ ion is represented by the
Q36: The Lewis structure of the CO<sub>3</sub><sup>2-</sup> ion
Q37: What is the electron configuration for the
Q38: The strength of a _ bond is