Multiple Choice
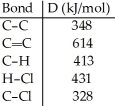
Using the table of bond dissociation energies,the ΔH for the following gas-phase reaction is ________ kJ. 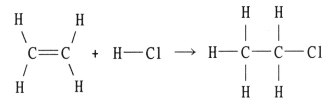
A) -44
B) 38
C) 304
D) 2134
E) -38
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q136: The reaction below is used to produce
Q137: From the information given below,calculate the heat
Q138: Which of the following has eight valence
Q139: Most transition metals do not form ions
Q140: How many valence electrons are in the
Q141: Using the table of average bond energies
Q142: The greater the lattice energy,the greater the
Q143: Based on the octet rule,magnesium most likely
Q144: What is the electron configuration for the
Q145: Which of the following has the bonds