Multiple Choice
A sample of an unknown volatile liquid was injected into a Dumas flask 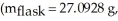
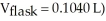 and heated until no visible traces of the liquid could be found.The flask and its contents were then rapidly cooled and reweighed
and heated until no visible traces of the liquid could be found.The flask and its contents were then rapidly cooled and reweighed 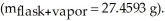 The atmospheric pressure and temperature during the experiment were 0.976 atm and 18.0 °C,respectively.The unknown volatile liquid was ________.
The atmospheric pressure and temperature during the experiment were 0.976 atm and 18.0 °C,respectively.The unknown volatile liquid was ________.
A) C6H12
B) C6H14
C) C7H14
D) C7H16
E) C6H6
Correct Answer:

Verified
Correct Answer:
Verified
Q97: A fixed amount of gas at 25.0
Q98: A gas mixture of N<sub>2</sub> and H<sub>2</sub>
Q99: A sample of CO<sub>2</sub> gas (3.0 mol)effused
Q100: A flask contains a mixture of N<sub>2</sub>
Q101: What volume (L)of fluorine gas is required
Q103: A sample of an ideal gas (3.00
Q104: A gas mixture of Xe,Ne,and Ar has
Q105: Of the following,_ is a correct statement
Q106: How many moles of an unknown gas
Q107: What is the density of carbon dioxide