Multiple Choice
A fixed amount of gas at 25.0 °C occupies a volume of 10.0 L when the pressure is 751 torr.Use Boyle's law to calculate the pressure (torr) when the volume is reduced to 7.25 L at a constant temperature of 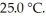
A) 1.04 × 103
B) 0.097
C) 5.44 × 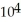
D) 544
E) 1.36
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q92: If 50.75 g of a gas occupies
Q93: 760 torr is equivalent to _ mm
Q94: A balloon originally had a volume of
Q95: The average kinetic energy of the particles
Q96: Two deviations of real gases from ideal
Q98: A gas mixture of N<sub>2</sub> and H<sub>2</sub>
Q99: A sample of CO<sub>2</sub> gas (3.0 mol)effused
Q100: A flask contains a mixture of N<sub>2</sub>
Q101: What volume (L)of fluorine gas is required
Q102: A sample of an unknown volatile liquid