Multiple Choice
A gas vessel is attached to an open-end manometer containing a nonvolatile liquid of density 0.791 g/mL as shown below. 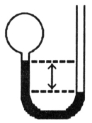 The difference in heights of the liquid in the two sides of the manometer is 43.4 cm when the atmospheric pressure is 755 mm Hg.Given that the density of mercury is 13.6 g/mL,the pressure of the enclosed gas is ________ atm.
The difference in heights of the liquid in the two sides of the manometer is 43.4 cm when the atmospheric pressure is 755 mm Hg.Given that the density of mercury is 13.6 g/mL,the pressure of the enclosed gas is ________ atm.
A) 1.03
B) 0.967
C) 0.993
D) 0.990
E) 0.987
Correct Answer:

Verified
Correct Answer:
Verified
Q124: How many moles of gas are there
Q125: Calculate the density of hydrogen gas (in
Q126: A gas vessel is attached to an
Q127: The effusion rate of a gas is
Q128: The amount of gas that occupies 36.52
Q130: The temperature of an ideal gas at
Q131: The density of nitric oxide (NO)gas at
Q132: Sodium hydride reacts with excess water to
Q133: Gaseous mixtures _.<br>A)can only contain molecules<br>B)are all
Q134: Sulfur dioxide (12.4 g)and carbon dioxide (12.4