Multiple Choice
A gas vessel is attached to an open-end manometer filled with a nonvolatile liquid of density 0.993 g/mL as shown below. 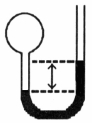 The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg.Given that the density of mercury is 13.6 g/mL,the pressure of the enclosed gas is ________ atm.
The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg.Given that the density of mercury is 13.6 g/mL,the pressure of the enclosed gas is ________ atm.
A) 1.05
B) 1.01
C) 0.976
D) 0.993
E) 1.08
Correct Answer:

Verified
Correct Answer:
Verified
Q121: Which one of the following is a
Q122: According to kinetic-molecular theory,in which of the
Q123: Of the following,_ has a strong acrid
Q124: How many moles of gas are there
Q125: Calculate the density of hydrogen gas (in
Q127: The effusion rate of a gas is
Q128: The amount of gas that occupies 36.52
Q129: A gas vessel is attached to an
Q130: The temperature of an ideal gas at
Q131: The density of nitric oxide (NO)gas at