Multiple Choice
A solution is prepared by dissolving 23.7 g of CaCl2 in 375 g of water.The density of the resulting solution is 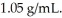 The concentration of CaCl2 in this solution is ________ molal.
The concentration of CaCl2 in this solution is ________ molal.
A) 0.214
B) 0.569
C) 5.70
D) 63.2
E) 1.76
Correct Answer:

Verified
Correct Answer:
Verified
Q68: The dissolution of water in octane (C<sub>8</sub>H<sub>18</sub>)is
Q69: What is the molarity of phosphoric acid
Q70: A water sample tested positive for lead
Q71: A solution with a solute concentration greater
Q72: As the concentration of a solute in
Q74: A mixture containing 33.0 g of an
Q75: A solution is prepared by dissolving 15.0
Q76: What is the molarity of sodium chloride
Q77: The concentration of urea in a solution
Q78: A solution at <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="A solution