Multiple Choice
A mixture containing 33.0 g of an unknown liquid and 230.0 g of water has a freezing point of -1.12 °C.Given 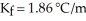 for water,what is the molar mass (g/mol) of the unknown liquid?
for water,what is the molar mass (g/mol) of the unknown liquid?
A) 54.8
B) 0.602
C) 143
D) 238
E) 138
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q69: What is the molarity of phosphoric acid
Q70: A water sample tested positive for lead
Q71: A solution with a solute concentration greater
Q72: As the concentration of a solute in
Q73: A solution is prepared by dissolving 23.7
Q75: A solution is prepared by dissolving 15.0
Q76: What is the molarity of sodium chloride
Q77: The concentration of urea in a solution
Q78: A solution at <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="A solution
Q79: Pairs of liquids that will mix in