Multiple Choice
The Henry's law constant for helium gas in water at 30 °C is 3.70 × 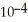 M/atm.When the partial pressure of helium above a sample of water is 0.400 atm,the concentration of helium in the water is ________ M.
M/atm.When the partial pressure of helium above a sample of water is 0.400 atm,the concentration of helium in the water is ________ M.
A) 9.25 × 10-4
B) 1.08 × 103
C) 0.800
D) 1.48 × 10-4
E) 3.70 × 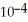
Correct Answer:

Verified
Correct Answer:
Verified
Q59: Which of the following aqueous solutions will
Q60: Compounds composed of a salt and water
Q61: The freezing point of ethanol ( <img
Q62: Hydration is a specific example of the
Q63: What is the molality of a 10.0%
Q65: What is the mole fraction of phosphoric
Q66: Which produces the greatest number of ions
Q67: What is the mole fraction of hydrochloric
Q68: The dissolution of water in octane (C<sub>8</sub>H<sub>18</sub>)is
Q69: What is the molarity of phosphoric acid