Multiple Choice
The freezing point of ethanol ( 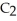
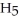 OH) is -114.6 °C.The molal freezing point depression constant for ethanol is 2.00 °C/m.What is the freezing point (°C) of a solution prepared by dissolving 50.0 g of glycerin
OH) is -114.6 °C.The molal freezing point depression constant for ethanol is 2.00 °C/m.What is the freezing point (°C) of a solution prepared by dissolving 50.0 g of glycerin 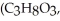 a nonelectrolyte) in 200.0 g of ethanol?
a nonelectrolyte) in 200.0 g of ethanol?
A) -115.0
B) -5.43
C) -132.3
D) -120.0
E) -114.6
Correct Answer:

Verified
Correct Answer:
Verified
Q56: An aqueous solution with a concentration of
Q57: The solubility of nitrogen gas at 25
Q58: Which one of the following is least
Q59: Which of the following aqueous solutions will
Q60: Compounds composed of a salt and water
Q62: Hydration is a specific example of the
Q63: What is the molality of a 10.0%
Q64: The Henry's law constant for helium gas
Q65: What is the mole fraction of phosphoric
Q66: Which produces the greatest number of ions