Multiple Choice
What is the solubility concentration (M) of nitrogen gas in 25 °C water and at a nitrogen pressure of 3.5 atm? The solubility of nitrogen gas in water at 25 °C and a nitrogen pressure of 1.0 atm is 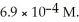
A) 0.00086
B) 120
C) 0.0037
D) 3.5
E) 0.0024
Correct Answer:

Verified
Correct Answer:
Verified
Q133: 13.3 g of benzene (C<sub>6</sub>H<sub>6</sub>)is dissolved in
Q134: Of the following,a 0.2 M aqueous solution
Q135: What is the formula weight of iron(III)chloride
Q136: What is the chloride ion concentration (M)in
Q137: The solubility of MnSO<sub>4</sub> monohydrate in water
Q139: Which one of the following solutes has
Q140: The concentration of CO<sub>2</sub> in a soft
Q141: What is the molality of a 35.0%
Q142: Which of the following liquids will have
Q143: What is the freezing point (°C)of a