Multiple Choice
What is the freezing point (°C) of a solution prepared by dissolving 11.3 g of Ca(NO3) 2 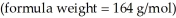 in 115 g of water? The molal freezing point depression constant for water is
in 115 g of water? The molal freezing point depression constant for water is 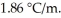 (Assume 100% ionization of Ca(NO3) 2.)
(Assume 100% ionization of Ca(NO3) 2.)
A) -3.34
B) -1.11
C) 3.34
D) 1.11
E) 0.00
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q138: What is the solubility concentration (M)of nitrogen
Q139: Which one of the following solutes has
Q140: The concentration of CO<sub>2</sub> in a soft
Q141: What is the molality of a 35.0%
Q142: Which of the following liquids will have
Q144: Of the following,a 0.1 M aqueous solution
Q145: The ideal value of i (van't Hoff
Q146: George is making spaghetti for dinner.He places
Q147: What is the molality (m)of a solution
Q148: What is the vapor pressure (mm Hg)of