Multiple Choice
A solution contains 30 ppm of some heavy metal and the density of the solution is 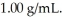 This means that ________.
This means that ________.
A) 100 g of the solution contains 30 g of heavy metal
B) 100 g of the solution contains 30 mg of heavy metal
C) the solution is 30% by mass of heavy metal
D) there are 30 mg of the heavy metal in 1.0 L of this solution
E) the molarity of the solution is 30 M
Correct Answer:

Verified
Correct Answer:
Verified
Q119: A 17.2 g sample of potassium chlorate
Q120: The ratio of the actual value of
Q121: What is the molality of ammonium chloride
Q122: Which one of the following concentration units
Q123: Which one of the following substances is
Q125: How much sodium nitrate (g)is in a
Q126: Which one of the following is most
Q127: The _ is a phenomenon used to
Q128: A solution contains 39% phosphoric acid by
Q129: What is the % by mass of