Multiple Choice
What is the molality of ammonium chloride in a 3.95 M ammonium chloride aqueous solution at 20 °C? Density of the solution is 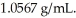
A) 0.0780
B) 3.95
C) 0.268
D) 20.00
E) 4.67
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q116: A solution contains 11% by mass of
Q117: A solution is prepared by dissolving 0.60
Q118: Calculate the molarity of a 17.5% (by
Q119: A 17.2 g sample of potassium chlorate
Q120: The ratio of the actual value of
Q122: Which one of the following concentration units
Q123: Which one of the following substances is
Q124: A solution contains 30 ppm of some
Q125: How much sodium nitrate (g)is in a
Q126: Which one of the following is most