Multiple Choice
What is the mole fraction of N 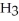 in a solution prepared by dissolving 16.0 g of N
in a solution prepared by dissolving 16.0 g of N 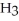 in 250.0 g of water? The density of the resulting solution is 0.974 g/mL.
in 250.0 g of water? The density of the resulting solution is 0.974 g/mL.
A) 0.0640
B) 0.940
C) 0.0635
D) 0.922
E) 16.8
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q110: Colligative properties of solutions include all of
Q111: Which one of the following is most
Q112: A solution is prepared by dissolving 23.7
Q113: A 30.0 g sample of potassium nitrate
Q114: The mole fraction of He in a
Q116: A solution contains 11% by mass of
Q117: A solution is prepared by dissolving 0.60
Q118: Calculate the molarity of a 17.5% (by
Q119: A 17.2 g sample of potassium chlorate
Q120: The ratio of the actual value of