Multiple Choice
A solution is prepared by dissolving 0.60 g of nicotine (a nonelectrolyte) in water to make 12 mL of solution.The osmotic pressure of the solution is 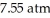 at 25 °C.The molecular weight of nicotine is
at 25 °C.The molecular weight of nicotine is 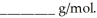
A) 28
B) 43
C) 50
D) 160
E) 0.60
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q112: A solution is prepared by dissolving 23.7
Q113: A 30.0 g sample of potassium nitrate
Q114: The mole fraction of He in a
Q115: What is the mole fraction of N
Q116: A solution contains 11% by mass of
Q118: Calculate the molarity of a 17.5% (by
Q119: A 17.2 g sample of potassium chlorate
Q120: The ratio of the actual value of
Q121: What is the molality of ammonium chloride
Q122: Which one of the following concentration units