Multiple Choice
What is the molality (m) of a solution containing 5.16 g of C6H12O6 in 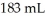 of water? The density of water is
of water? The density of water is 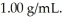
A) 0.313
B) 0.156
C) 0.0782
D) 28.2
E) 0.00524
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q142: Which of the following liquids will have
Q143: What is the freezing point (°C)of a
Q144: Of the following,a 0.1 M aqueous solution
Q145: The ideal value of i (van't Hoff
Q146: George is making spaghetti for dinner.He places
Q148: What is the vapor pressure (mm Hg)of
Q149: A solution is prepared by dissolving 24.7
Q150: The most likely van't Hoff factor for
Q151: The concentration of urea (MW = 60.0
Q152: Hydrophobic colloids _.<br>A)are those that contain water<br>B)can