Multiple Choice
Calculate the freezing point of a 0.09500 m aqueous solution of glucose.The molal freezing-point-depression constant of water is 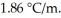
A) 0.0475
B) 0.106
C) -0.0562
D) -0.177
E) -0.354
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: Which component of air is the primary
Q7: What is the mole fraction of HCl
Q8: How many grams of solution are present
Q9: The process of solute particles being surrounded
Q10: The vapor pressure of pure ethanol at
Q12: Which of the following statements is false?<br>A)Nonpolar
Q13: All of the following are considered to
Q14: What is the mole fraction of nitric
Q15: Calculate the mole fraction of phosphoric acid
Q16: A supersaturated solution _.<br>A)is one with more