Multiple Choice
What is the mole fraction of HCl in a solution that is prepared by dissolving 25.5 g of HCl in 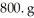 of water? The density of the solution is 1.0 g/mL.
of water? The density of the solution is 1.0 g/mL.
A) 0.0155
B) 0.0319
C) 0.984
D) 0.874
E) 32.0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: A solution is prepared by dissolving 15.8
Q3: The vapor pressure of pure water at
Q4: Calculate the vapor pressure of a solution
Q5: A solution is prepared by dissolving 25.0
Q6: Which component of air is the primary
Q8: How many grams of solution are present
Q9: The process of solute particles being surrounded
Q10: The vapor pressure of pure ethanol at
Q11: Calculate the freezing point of a 0.09500
Q12: Which of the following statements is false?<br>A)Nonpolar