Multiple Choice
Calculate the freezing point of a solution containing 20 grams of KCl and 2200.0 grams of water.The molal-freezing-point-depression constant ( 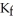 ) for water is
) for water is 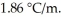 (Assume 100% ionization of KCl.)
(Assume 100% ionization of KCl.)
A) -0.45 °C
B) +0.45 °C
C) -0.23 °C
D) +0.23 °C
E) 1.23 °C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: Which of the following substances is more
Q20: The mole fraction of NaCl in water
Q21: After swimming in the ocean for several
Q22: Pressure has an appreciable effect on the
Q23: In a saturated solution of a salt
Q25: What is the molality of a 36.1%
Q26: The molarity of urea (MW = 60.0
Q27: A 0.200 m solution of which one
Q28: An aqueous solution of a soluble compound
Q29: Emulsifying agents typically have a hydrophobic end