Multiple Choice
An aqueous solution of a soluble compound (a nonelectrolyte) is prepared by dissolving 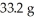 of the compound in sufficient water to form
of the compound in sufficient water to form 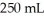 of solution.The solution has an osmotic pressure of 1.2 atm at
of solution.The solution has an osmotic pressure of 1.2 atm at 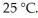 What is the molar mass (g/mole) of the compound?
What is the molar mass (g/mole) of the compound?
A) 1.0 × 103
B) 2.7 × 103
C) 2.3 × 102
D) 6.8 × 102
E) 28
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q23: In a saturated solution of a salt
Q24: Calculate the freezing point of a solution
Q25: What is the molality of a 36.1%
Q26: The molarity of urea (MW = 60.0
Q27: A 0.200 m solution of which one
Q29: Emulsifying agents typically have a hydrophobic end
Q30: The concentration of lead nitrate (Pb(N <img
Q31: What is the molality of a 10.0%
Q32: A solution is prepared by dissolving 2.00
Q33: Which produces the greatest number of ions