Multiple Choice
A possible mechanism for the overall reaction Br2 (g) + 2NO (g) → 2NOBr (g)
Is
Step 1) NO (g) + Br2 (g) 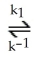 NOBr2 (g) (fast)
NOBr2 (g) (fast)
Step 2) NOBr2 (g) + NO (g) 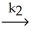 2NOBr (slow)
2NOBr (slow)
What is the rate determining step for this reaction?
A) step 2
B) reverse of step 1
C) both steps 1 and 2
D) step 1
E) reverse of step 2
Correct Answer:

Verified
Correct Answer:
Verified
Q28: Heterogeneous catalysts have different phases from reactants.
Q29: What is the rate constant (s<sup>-1</sup>)for this
Q30: A first-order reaction has a rate constant
Q31: What is the rate constant ( <img
Q32: Of the units below,_ are appropriate for
Q34: The average rate disappearance of A between
Q35: The average rate of disappearance of I<sup>-</sup>
Q36: A particular first-order reaction has a rate
Q37: The mechanism for formation of the product
Q38: Of the following,only _ is a valid