Multiple Choice
A first-order reaction has a rate constant of 0.33 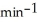 .It takes ________ min for the reactant concentration to decrease from 0.13 M to 0.066 M.
.It takes ________ min for the reactant concentration to decrease from 0.13 M to 0.066 M.
A) 0.085
B) 0.13
C) 0.89
D) 2.4
E) 2.1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q25: Which of the following statements describes why
Q26: Define a homogeneous catalyst.
Q27: What is the concentration (M)of S<sub>2</sub>O<sub>8</sub><sup>2-</sup> remaining
Q28: Heterogeneous catalysts have different phases from reactants.
Q29: What is the rate constant (s<sup>-1</sup>)for this
Q31: What is the rate constant ( <img
Q32: Of the units below,_ are appropriate for
Q33: A possible mechanism for the overall reaction
Q34: The average rate disappearance of A between
Q35: The average rate of disappearance of I<sup>-</sup>