Multiple Choice
The decomposition of 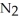
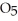 in solution in carbon tetrachloride proceeds via the reaction 2N2O5 (soln) → 4NO2 (soln) + O2 (soln)
in solution in carbon tetrachloride proceeds via the reaction 2N2O5 (soln) → 4NO2 (soln) + O2 (soln)
The reaction is first order and has a rate constant of 4.82 × 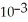
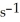 at 64 °C.If the reaction is initiated with 0.072 mol in a 1.00-L vessel,how many moles remain after 151 s?
at 64 °C.If the reaction is initiated with 0.072 mol in a 1.00-L vessel,how many moles remain after 151 s?
A) 0.067
B) 0.074
C) 0.035
D) 9.6
E) 1.6 × 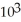
Correct Answer:

Verified
Correct Answer:
Verified
Q111: Of the following,all are valid units for
Q112: The isomerization of methylisonitrile to acetonitrile CH<sub>3</sub>NC
Q113: The initial concentration of reactant in a
Q114: Which one of the following is not
Q115: At elevated temperatures,dinitrogen pentoxide decomposes to nitrogen
Q117: The overall order of a reaction is
Q118: In general,as temperature goes up,reaction rate _.<br>A)goes
Q119: The reaction A → B is first
Q120: In the energy profile of a reaction,the
Q121: The rate law for this reaction is