Multiple Choice
The initial concentration of reactant in a first-order reaction is 0.27 M and has a rate constant of 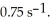 What is the concentration (mol/L) of reactant after
What is the concentration (mol/L) of reactant after 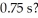
A) 0.99
B) 1.7
C) 0.074
D) 0.15
E) 0.47
Correct Answer:

Verified
Correct Answer:
Verified
Q108: For a zero-order reaction,a plot of _
Q109: Biological catalysts are referred to as _.
Q110: The reaction A (aq)→ B (aq)is first
Q111: Of the following,all are valid units for
Q112: The isomerization of methylisonitrile to acetonitrile CH<sub>3</sub>NC
Q114: Which one of the following is not
Q115: At elevated temperatures,dinitrogen pentoxide decomposes to nitrogen
Q116: The decomposition of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The decomposition
Q117: The overall order of a reaction is
Q118: In general,as temperature goes up,reaction rate _.<br>A)goes