Multiple Choice
The rate constant for a second-order reaction is 0.13 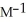
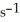 .If the initial concentration of reactant is
.If the initial concentration of reactant is 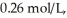 it takes ________ s for the concentration to decrease to
it takes ________ s for the concentration to decrease to 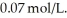
A) 0.017
B) 1.4
C) 14
D) 80.
E) 10.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: What is the magnitude of k for
Q17: For the elementary reaction NO<sub>3</sub> + CO
Q18: Which of the following is correct regarding
Q19: The reaction 2NO<sub>2</sub> → 2NO + O<sub>2</sub><br>Follows
Q20: The average rate of disappearance of A
Q22: Which of the following is true?<br>A)If we
Q23: According to the collision model,reaction rates are
Q24: A reaction was found to be zero
Q25: Which of the following statements describes why
Q26: Define a homogeneous catalyst.