Multiple Choice
What is the magnitude of k for a first-order reaction at 75.0 °C if 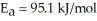 and rate constant is 1.35 ×
and rate constant is 1.35 × 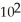
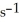 at 25.0 °C?
at 25.0 °C?
A) 3.36 × 104
B) 3.85 × 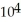
C) 745
D) 4.28 × 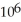
E) 1.36 × 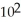
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Of the following,_ will lower the activation
Q12: Fe and Mo are transition metals found
Q13: The reaction CH<sub>3</sub>-N≡C → CH<sub>3</sub>-C≡N<br>Is a first-order
Q14: A compound decomposes by a first-order process.If
Q15: At elevated temperatures,dinitrogen pentoxide decomposes to nitrogen
Q17: For the elementary reaction NO<sub>3</sub> + CO
Q18: Which of the following is correct regarding
Q19: The reaction 2NO<sub>2</sub> → 2NO + O<sub>2</sub><br>Follows
Q20: The average rate of disappearance of A
Q21: The rate constant for a second-order reaction