Multiple Choice
The expression for 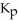 for the reaction below is ________. 4CuO (s) + CH4 (g)
for the reaction below is ________. 4CuO (s) + CH4 (g)  CO2 (g) + 4Cu (s) + 2H2O (g)
CO2 (g) + 4Cu (s) + 2H2O (g)
A) 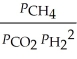
B) 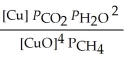
C) 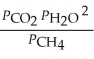
D) 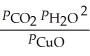
E) 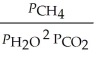
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q25: Le Châtelier's principle states that if a
Q26: How is the reaction quotient used to
Q27: Phosphorous trichloride and phosphorous pentachloride equilibrate in
Q28: In the coal-gasification process,carbon monoxide reacts with
Q29: The equilibrium constant for the gas phase
Q31: The value of K<sub>eq</sub> for the equilibrium
Q32: The effect of a catalyst on a
Q33: At 900.0 K,the equilibrium constant (K<sub>p</sub>)for the
Q34: If a reaction is exothermic,_ the reaction
Q35: The equilibrium constant for the gas phase