Multiple Choice
At 900.0 K,the equilibrium constant (Kp) for the following reaction is 0.345. 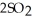 +
+ 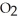 (g) →
(g) → 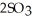 (g) At equilibrium,the partial pressure of
(g) At equilibrium,the partial pressure of 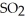 is 36.9 atm and that of
is 36.9 atm and that of 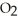 is 16.8 atm.The partial pressure of
is 16.8 atm.The partial pressure of 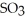 is ________ atm.
is ________ atm.
A) 88.8
B) 3.89 × 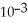
C) 214
D) 5.57 × 10-4
E) 42.4
Correct Answer:

Verified
Correct Answer:
Verified
Q28: In the coal-gasification process,carbon monoxide reacts with
Q29: The equilibrium constant for the gas phase
Q30: The expression for <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The expression
Q31: The value of K<sub>eq</sub> for the equilibrium
Q32: The effect of a catalyst on a
Q34: If a reaction is exothermic,_ the reaction
Q35: The equilibrium constant for the gas phase
Q36: The equilibrium constant (K<sub>p</sub>)for the interconversion of
Q37: Which of the following statements is true?<br>A)Q
Q38: The equilibrium constant for reaction 1 is