Multiple Choice
Dinitrogentetraoxide partially decomposes into nitrogen dioxide.A 1.00-L flask is charged with 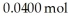 of N2O4.At equilibrium at 373 K,0.0055 mol of N2O4 remains.Keq for this reaction is ________.
of N2O4.At equilibrium at 373 K,0.0055 mol of N2O4 remains.Keq for this reaction is ________.
A) 2.2 × 10-4
B) 13
C) 0.22
D) 0.87
E) 0.022
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q59: Which of the following expressions is the
Q60: The value of K<sub>eq</sub> for the following
Q61: The equilibrium constant for the gas phase
Q62: Define the reaction quotient.
Q63: Le Châtelier's principle states that if a
Q65: What role did Karl Bosch play in
Q66: The value of K<sub>eq</sub> for the equilibrium
Q67: The equilibrium constant for the gas phase
Q68: Nitrosyl bromide decomposes according to the following
Q69: Consider the following reaction at equilibrium. 2CO<sub>2</sub>