Multiple Choice
Nitrosyl bromide decomposes according to the following equation. 2NOBr (g)  2NO (g) +
2NO (g) + 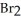 (g)
(g)
A sample of NOBr (0.64 mol) was placed in a 1.00-L flask containing no NO or 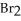 .At equilibrium the flask contained
.At equilibrium the flask contained 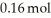 of NOBr.How many moles of NO and
of NOBr.How many moles of NO and 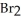 ,respectively,are in the flask at equilibrium?
,respectively,are in the flask at equilibrium?
A) 0.48, 0.24
B) 0.48, 0.48
C) 0.16, 0.08
D) 0.16, 0.16
E) 0.24, 0.42
Correct Answer:

Verified
Correct Answer:
Verified
Q63: Le Châtelier's principle states that if a
Q64: Dinitrogentetraoxide partially decomposes into nitrogen dioxide.A 1.00-L
Q65: What role did Karl Bosch play in
Q66: The value of K<sub>eq</sub> for the equilibrium
Q67: The equilibrium constant for the gas phase
Q69: Consider the following reaction at equilibrium. 2CO<sub>2</sub>
Q70: The value of K<sub>eq</sub> for the equilibrium
Q71: Given the following reaction at equilibrium at
Q72: At equilibrium,_.<br>A)all chemical reactions have ceased<br>B)the rates
Q73: At 24°C,K<sub>p</sub> = 0.080 for the equilibrium: