Multiple Choice
Carbon monoxide and chlorine gas react to produce COCl2 gas.The 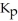 for the reaction is 1.49 × 108 at 100.0 °C: In an equilibrium mixture of the three gases,
for the reaction is 1.49 × 108 at 100.0 °C: In an equilibrium mixture of the three gases, 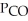 =
= 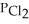 = 7.70 × 10-4 atm.The partial pressure of the product,phosgene (
= 7.70 × 10-4 atm.The partial pressure of the product,phosgene ( 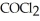 ) ,is ________ atm.
) ,is ________ atm.
A) 2.51 × 1014
B) 3.98 × 10-15
C) 1.15 × 105
D) 8.83 × 101
E) 1.92 × 1011
Correct Answer:

Verified
Correct Answer:
Verified
Q6: The K<sub>eq</sub> for the equilibrium below is
Q7: The expression of K<sub>eq</sub> for the following
Q8: At elevated temperatures,molecular hydrogen and molecular bromine
Q9: If the reaction quotient Q for a
Q10: Hydrogen and iodine gas react to produce
Q12: At 200 °C,the equilibrium constant (K<sub>p</sub>)for the
Q13: The K<sub>eq</sub> for the equilibrium below is
Q14: At 400 K,the equilibrium constant for the
Q15: In which of the following reactions would
Q16: The equilibrium-constant expression depends on the _