Multiple Choice
In which of the following reactions would increasing pressure at constant temperature change the concentrations of reactants and products,based on Le Châteliers principle?
A) N2 (g) + 3H2 (g)  2NH3 (g)
2NH3 (g)
B) N2O4 (g)  2NO2 (g)
2NO2 (g)
C) N2 (g) + 2O2 (g)  2NO2 (g)
2NO2 (g)
D) 2N2 (g) + O2 (g)  2
2 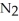 O (g)
O (g)
E) all of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Hydrogen and iodine gas react to produce
Q11: Carbon monoxide and chlorine gas react to
Q12: At 200 °C,the equilibrium constant (K<sub>p</sub>)for the
Q13: The K<sub>eq</sub> for the equilibrium below is
Q14: At 400 K,the equilibrium constant for the
Q16: The equilibrium-constant expression depends on the _
Q17: Consider the following reaction at equilibrium: 2S<br>(g)+
Q18: The equilibrium-constant expression for the reaction Ti
Q19: If a reaction is endothermic,_ the reaction
Q20: If Reaction A + Reaction B =