Multiple Choice
Consider the following reaction at equilibrium: 2S
(g) + C:\Users\User\Dropbox\Quizplus Parsing Documents\To Be Parsed\Not Complete Tables\Chemistry\TB1194,Chemistry The Central Science 14th Edition by Theodore E.Brown\TB1194,Chemistry The Central Science 14th Edition by Theodore E.Brown\Images\C10074 (15) _205.jpg
(g) C:\Users\User\Dropbox\Quizplus Parsing Documents\To Be Parsed\Not Complete Tables\Chemistry\TB1194,Chemistry The Central Science 14th Edition by Theodore E.Brown\TB1194,Chemistry The Central Science 14th Edition by Theodore E.Brown\Images\C10074 (15) _206.jpg
2S C:\Users\User\Dropbox\Quizplus Parsing Documents\To Be Parsed\Not Complete Tables\Chemistry\TB1194,Chemistry The Central Science 14th Edition by Theodore E.Brown\TB1194,Chemistry The Central Science 14th Edition by Theodore E.Brown\Images\C10074 (15) _207.jpg
(g) ΔH° = -99 kJ
Le Châtelier's principle predicts that a(n) increase in temperature will result in ________.
A) an increase in the partial pressure of O2
B) a decrease in the partial pressure of O2
C) a decrease in the partial pressure of SO2
D) a(n) increase in 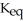
E) no changes in equilibrium partial pressures
Correct Answer:

Verified
Correct Answer:
Verified
Q12: At 200 °C,the equilibrium constant (K<sub>p</sub>)for the
Q13: The K<sub>eq</sub> for the equilibrium below is
Q14: At 400 K,the equilibrium constant for the
Q15: In which of the following reactions would
Q16: The equilibrium-constant expression depends on the _
Q18: The equilibrium-constant expression for the reaction Ti
Q19: If a reaction is endothermic,_ the reaction
Q20: If Reaction A + Reaction B =
Q21: Based on Le Châtelier's principle,increasing pressure at
Q22: Which one of the following is true