Multiple Choice
The hydride ion, 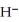 ,is a stronger base than the hydroxide ion,O
,is a stronger base than the hydroxide ion,O 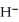 .The product(s) of the reaction of hydride ion with water is/are ________.
.The product(s) of the reaction of hydride ion with water is/are ________.
A) H3O+ (aq)
B) OH- (aq) + H2 (g)
C) OH- (aq) + 2H+ (aq)
D) no reaction occurs
E) H2O2 (aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q57: A Br∅nsted-Lowry base is defined as a
Q58: Which solution will be the most basic?<br>A)0.10
Q59: What is the pOH of a sodium
Q60: Using the data in the table,which of
Q61: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The of
Q63: Ammonia is a _.<br>A)weak acid<br>B)strong base<br>C)weak base<br>D)strong
Q64: Of the following,which is the weakest acid?<br>A)HPO<sub>3</sub><sup>-</sup><br>B)H<sub>3</sub>PO<sub>4</sub><br>C)H<sub>2</sub>PO<sub>4</sub><sup>-</sup><br>D)HPO<sub>4</sub><sup>-</sup><br>E)The
Q65: Using the data in the table,which of
Q66: K<sub>a</sub> for arsenic acic,HAsO<sub>4</sub><sup>2-</sup>,is 7.5 × 10<sup>-12</sup>.What
Q67: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The of