Multiple Choice
Using the data in the table,which of the conjugate bases below is the weakest base? 
A) OAc-
B) C7H5O2-
C) NO2-
D) 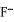
E) OAc- and C7H5O2-
Correct Answer:

Verified
Correct Answer:
Verified
Q55: K<sub>a</sub> for HA is 4.9 × 10<sup>-10</sup>.What
Q56: A substance that is capable of acting
Q57: A Br∅nsted-Lowry base is defined as a
Q58: Which solution will be the most basic?<br>A)0.10
Q59: What is the pOH of a sodium
Q61: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The of
Q62: The hydride ion, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The hydride
Q63: Ammonia is a _.<br>A)weak acid<br>B)strong base<br>C)weak base<br>D)strong
Q64: Of the following,which is the weakest acid?<br>A)HPO<sub>3</sub><sup>-</sup><br>B)H<sub>3</sub>PO<sub>4</sub><br>C)H<sub>2</sub>PO<sub>4</sub><sup>-</sup><br>D)HPO<sub>4</sub><sup>-</sup><br>E)The
Q65: Using the data in the table,which of