Multiple Choice
Of the acids in the table below,________ is the strongest acid. 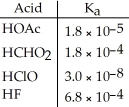
A) HOAc
B) HCHO2
C) HClO
D) HF
E) HOAc and HCHO2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q97: The K<sub>a</sub> of acetic acid (HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>)is <img
Q98: The pOH of a 0.25 M aqueous
Q99: Calculate the molarity of hydroxide ion in
Q100: A 0.14 M aqueous solution of the
Q101: Of the following substances,an aqueous solution of
Q103: In which of the following aqueous solutions
Q104: Which solution below has the highest concentration
Q105: A Br∅nsted-Lowry acid is defined as a
Q106: The acid-dissociation constants of phosphoric acid (H<sub>3</sub>PO<sub>4</sub>)are
Q107: What is the pH of a sodium