Multiple Choice
The acid-dissociation constants of phosphoric acid (H3PO4) are 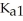 = 7.5 × 10-3,
= 7.5 × 10-3, 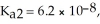 and Ka3 = 4.2 × 10-13 at 25.0 °C.What is the pH of a 2.5 M aqueous solution of phosphoric acid?
and Ka3 = 4.2 × 10-13 at 25.0 °C.What is the pH of a 2.5 M aqueous solution of phosphoric acid?
A) 1.82
B) 0.40
C) 2.51
D) 0.86
E) 0.13
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q101: Of the following substances,an aqueous solution of
Q102: Of the acids in the table below,_
Q103: In which of the following aqueous solutions
Q104: Which solution below has the highest concentration
Q105: A Br∅nsted-Lowry acid is defined as a
Q107: What is the pH of a sodium
Q108: The pH of a 0.25 M aqueous
Q109: The acid-dissociation constant,K<sub>a</sub>,for an unknown acid HA
Q110: An aqueous solution of NaF is prepared
Q111: Using the data in the table,which of