Multiple Choice
The Ka of hypochlorous acid (HClO) is 3.0 × 10-8 at 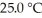 .What is the percent ionization of hypochlorous acid in a
.What is the percent ionization of hypochlorous acid in a 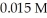 aqueous solution of HClO at
aqueous solution of HClO at 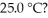
A) 4.5 × 10-8
B) 14
C) 2.1 × 10-5
D) 0.14
E) 1.4 × 10-3
Correct Answer:

Verified
Correct Answer:
Verified
Q122: The conjugate base of HPO<sub>4</sub><sup>2-</sup> is _.<br>A)PO<sub>4</sub><sup>3-</sup><br>B)H<sub>2</sub>PO<sub>4</sub><br>C)H<sub>3</sub>PO<sub>4</sub><br>D)H<sub>2</sub>PO<sub>4</sub><sup>-</sup><br>E)none
Q123: In the reaction<br>BF<sub>3</sub> + F<sup>-</sup> → BF<sub>4</sub><sup>-</sup><br>BF<sub>3</sub>
Q124: Which of the following aqueous solutions has
Q125: In acidic solution,_.<br>A)[H<sub>3</sub>O<sup>+</sup>] > [OH<sup>-</sup>]<br>B)[H<sub>3</sub>O<sup>+</sup>] = [OH<sup>-</sup>]<br>C)[H<sub>3</sub>O<sup>+</sup>]
Q126: Which of the following acids will be
Q128: What is the pOH of an aqueous
Q129: What is the pH of an aqueous
Q130: Which one of the following statements regarding
Q131: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The of
Q132: What is the conjugate acid of HCO<sub>3</sub><sup>-</sup>?<br>A)CO<sub>2</sub><sup>2-</sup><br>B)H<sub>2</sub>CO<sub>3</sub><br>C)HCO<sub>2</sub><sup>2-</sup><br>D)CO<sub>3</sub><sup>2-</sup><br>E)none