Multiple Choice
The 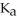 of hydrofluoric acid (HF) at 25.0 °C is 6.8 ×
of hydrofluoric acid (HF) at 25.0 °C is 6.8 × 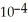 .What is the pH of a 0.45 M aqueous solution of HF?
.What is the pH of a 0.45 M aqueous solution of HF?
A) 4.05
B) 1.76
C) 3.64
D) 0.35
E) 1.41
Correct Answer:

Verified
Correct Answer:
Verified
Q126: Which of the following acids will be
Q127: The K<sub>a</sub> of hypochlorous acid (HClO)is 3.0
Q128: What is the pOH of an aqueous
Q129: What is the pH of an aqueous
Q130: Which one of the following statements regarding
Q132: What is the conjugate acid of HCO<sub>3</sub><sup>-</sup>?<br>A)CO<sub>2</sub><sup>2-</sup><br>B)H<sub>2</sub>CO<sub>3</sub><br>C)HCO<sub>2</sub><sup>2-</sup><br>D)CO<sub>3</sub><sup>2-</sup><br>E)none
Q133: HZ is a weak acid.An aqueous solution
Q134: Which of the following ions will act
Q135: The K<sub>a</sub> of hypochlorous acid (HClO)is <img
Q136: Of the following,_ is a weak acid.<br>A)HF<br>B)HCl<br>C)HBr<br>D)HNO<sub>3</sub><br>E)HClO<sub>4</sub>