Multiple Choice
Using the data in the table,which of the conjugate bases below is the strongest base? 
A) OAc-
B) C7H5O2-
C) NO2-
D) 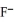
E) OAc- and C7H5 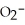
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q106: The acid-dissociation constants of phosphoric acid (H<sub>3</sub>PO<sub>4</sub>)are
Q107: What is the pH of a sodium
Q108: The pH of a 0.25 M aqueous
Q109: The acid-dissociation constant,K<sub>a</sub>,for an unknown acid HA
Q110: An aqueous solution of NaF is prepared
Q112: What is the pOH of an aqueous
Q113: The pH of a 0.25 M aqueous
Q114: The pH of a 0.15 M aqueous
Q115: The pH of a 0.25 M aqueous
Q116: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The for