Multiple Choice
The Ka of benzoic acid is 6.30 × 10-5.The pH of a buffer prepared by combining 50.0 mL of 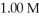 potassium benzoate and 50.0 mL of 1.00 M benzoic acid is ________.
potassium benzoate and 50.0 mL of 1.00 M benzoic acid is ________.
A) 1.705
B) 0.851
C) 3.406
D) 4.201
E) 2.383
Correct Answer:

Verified
Correct Answer:
Verified
Q81: The solubility of manganese (II)hydroxide (Mn(OH)<sub>2</sub>)is <img
Q82: The solubility of a slightly soluble salt
Q83: The addition of hydrofluoric acid and _
Q84: The solubility of lead (II)chloride (PbCl<sub>2</sub>)is <img
Q85: Calculate the pH of a solution prepared
Q87: Determine the K<sub>sp</sub> for magnesium hydroxide (Mg(OH)<sub>2</sub>)where
Q88: A buffer solution with a pH of
Q89: A 25.0 mL sample of 0.150 M
Q90: In which of the following aqueous solutions
Q91: Which solution has the greatest buffering capacity?<br>A)0.335